






Coloured glass is something that’s commonplace in our lives, from the green of wine and beer bottles, to the red, yellow, and green of traffic lights. The origin of these colours is something we don’t give a lot of thought to, but a range of different elements are responsible. This graphic takes a simple look at a few of these, and the colours they impart.

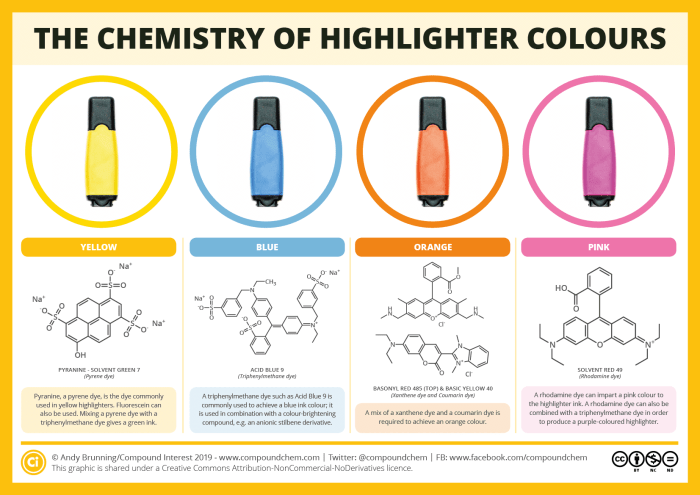
If you’re currently a student, then you’ll no doubt often make ample use of highlighters during revision. Even if your studying days are far behind, you probably still use them from time to time. But what are the chemicals behind their luminous colours? This graphic looks at some of the possible dyes that can be used.


There are a wide range of gemstones used in jewellery, with each having its own characteristic colour – or, in some cases, a range of colours. The origin of these colours has a chemical basis, and the precise colour can vary depending on the chemical composition of the gemstone. Interestingly, many minerals are actually colourless in their pure form, and it is the inclusion of impurities in their structure which leads to their colouration.


A previous post looked at the colours of transition metals, and the origin of their colours – this graphic, on the other hand, looks at how transition metals (and some non-transition metals) can be identified by the precipitates they form with sodium hydroxide and ammonia solutions. I’m going to keep the explanation of the reasons for the colour changes and precipitates fairly simple here, but I’ve provided links at the bottom of the page if you want to read about them in more detail.

This graphic looks at the colours of transition metal ions when they are in aqueous solution (in water), and also looks at the reason why we see coloured compounds and complexes for transition metals. This helps explain, for example, why rust (iron oxide) is an orange colour, and why the Statue of Liberty, made of copper, is no longer the shiny, metallic orange of copper, but a pale green colour given by the compound copper carbonate.