
In organic chemistry, isomers are molecules with the same molecular formula (i.e. the same number of atoms of each element), but different structural or spatial arrangements of the atoms within the molecule. The reason there are such a colossal number of organic compounds – more than 10 million – is in part down to isomerism. This graphic looks at the 5 main types of isomerism in organic molecules, with a more detailed explanation of each given below, as well as the reason why isomerism is important in our day-to-day lives.
STRUCTURAL ISOMERISM
Isomers can be split into two broad groups – structural (or constitutional) isomers, and stereoisomers. We’ll consider structural isomers first, which can be split again into three main subgroups: chain isomers, position isomers, and functional group isomers. Structural isomerism can quickly get quite out of hand in terms of the number of possible isomers; butane (four carbons) has two possible isomers, decane (ten carbons) has seventy-five, and a simple hydrocarbon containing 40 carbon atoms has an estimated 62,000,000,000 structural isomers.
Chain Isomers
Chain isomers are molecules with the same molecular formula, but different arrangements of the carbon ‘skeleton’. Organic molecules are based on chains of carbon atoms, and for many molecules this chain can be arranged differently: either as one, continuous chain, or as a chain with multiple side groups of carbons branching off. The name of the molecule can be changed to reflect this, but we’ll save the naming of molecules for another post. Obviously, there’s often more than one way of branching off groups of carbons from the main chain, which leads to the large numbers of possible isomers as the number of carbons in the molecule increases.
Position Isomers
Position isomers are based on the movement of a ‘functional group’ in the molecule. A functional group in organic chemistry is the part of a molecule that gives it its reactivity. There are a range of different functional groups, the more common of which were summarised in a previous post here. Nothing else about the molecule changes, simply where the functional group in it is, and the name simply alters slightly to indicate whereabouts in the molecule it is located.
Functional Isomers
Also referred to as functional group isomers, these are isomers where the molecular formula remains the same, but the type of functional group in the atom is changed. This is possible by rearranging the atoms within the molecule so that they’re bonded together in different ways. As an example, a standard straight-chain alkane (containing only carbon and hydrogen atoms) can have a functional group isomer that is a cycloalkane, which is simply the carbons bonded together in such a way that they form a ring. Different functional group isomers are possible for different functional groups.
STEREOISOMERISM
There are two main types of stereoisomerism – geometric isomerism, and optical isomerism. These, as the difference in name suggests, aren’t to do with any large scale rearrangements of the structure of molecules; instead, they involve different arrangements of parts of the molecule in space. They’re a little more complicated to think about than the structural isomers, so let’s have a look at each of them in turn.
Geometric Isomers
Geometric isomerism is actually a term that is ‘strongly discouraged’ by IUPAC (the International Union of Pure & Applied Chemistry), who prefer ‘cis-trans’, or ‘E-Z’ in the specific case of alkenes. However, ‘geometric isomerism’ is still consistently used in many A Level courses to refer to both, so for that reason I’ve used that name here.
This type of isomerism most frequently involves carbon carbon double bonds (shown by two lines joining each carbon instead of one). Rotation of these bonds is restricted, compared to single bonds, which can rotate freely. This means that, if there are two different atoms, or groups of atoms, attached to each carbon of the carbon carbon double bond, they can be arranged in different ways to give different molecules. These atoms or groups can be given ‘priorities’, with atoms with higher atomic numbers given higher priorities. If the highest priority groups for each carbon are on the same side of the molecule, that molecule is denoted as the ‘cis’ or ‘Z’ isomer. If they’re on opposite sites, it’s denoted as the ‘trans’ or ‘E’ isomer.

The two different nomenclatures are a little confusing – cis/trans is now less commonly used, with E/Z instead being favoured. E stands for ‘entgegen’ (‘opposite’ in german) whilst Z stands for ‘zusammen’ (‘together’ in german). The letter is simply added in brackets at the start of the molecule’s name in order to indicate which isomer it is.
Optical Isomers
Optical isomers are so named due to their effect on plane-polarised light, about which you can read more here, and come in pairs. They usually (although not always) contain a chiral centre – this is a carbon atom, with four different atoms (or groups of atoms) attached to it. These atoms or groups can be arranged differently around the central carbon, in such a way that the molecule can’t be rotated to make the two arrangements align. Since one arrangement can’t line up to look exactly like the other, we refer to them as ‘non-superimposable mirror images’ – one of the isomers is the mirror image of the other. Think of it like your hands – you can’t exactly superimpose one hand on top of the other, because your thumbs will stick out in opposite directions.
These can be allocated an identifying letter, in much the same way as with geometric isomerism. The groups around the carbon are given priorities, then the lowest priority group is oriented pointing away. Looking at the remaining groups, if they decrease in priority going in an anti-clockwise direction, it’s the S isomer (from the Latin ‘sinister’, meaning ‘left’). If they decrease in priority going in a clockwise direction, it’s the R isomer (from the Latin ‘rectus’, meaning ‘right’). Again, this letter is simply added in front of the isomer’s name in order to indicate which one it is.

There are other ways in which optical isomerism can be exhibited, but this is the simplest.
The Importance of Isomerism
As previously mentioned, isomers of the same molecule have the potential to have different physical or chemical properties. These differences can have some important implications.
Let’s look particularly at the case of optical isomerism. The two possible isomers can also be referred to as ‘enantiomers’ of each other. A prime, and well cited example of enantiomers with differing properties is that of the compound ‘carvone’. In its (R) form, it is found in mint leaves, and is the principle contributor to the aroma. However, in its S form, it is found in caraway seeds, and has a very different smell.
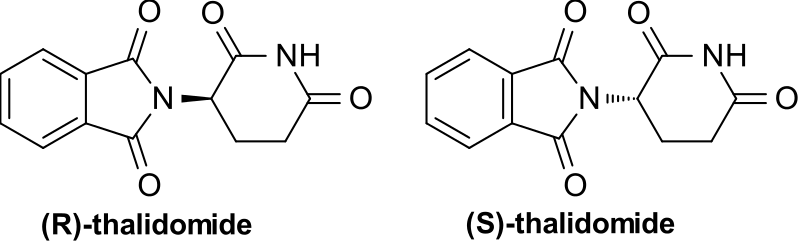
There can also be less benign differences. By far the most well known example here is that of thalidomide. This drug was prescribed in the 1950s and 60s to treat morning sickness in pregnant women; however, unknown then was that the (S) enantiomer could be transformed in the body into compounds that caused deformities in embryos. The two enantiomers also interconvert in the body, meaning that even if just the (R) enantiomer could be isolated, it would still produce the same effects. This emphasised the importance of testing all of the optical isomers of drugs for effects, and is part of the reason why present-day pharmaceuticals have to go through years of rigorous tests, to ensure that they are safe.
![]()
The graphic in this article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. See the site’s content usage guidelines.
References & Further Reading
- Molecule of the Day – Thalidomides
- Isomerism – ChemGuide





![Organic Functional Groups [2016]](https://i0.wp.com/www.compoundchem.com/wp-content/uploads/2014/01/Organic-Functional-Groups-2016.png?resize=350%2C288&ssl=1)
9 Comments
luisbrudna
my name is Luis Roberto Brudna Holzle, and I teach Chemistry at
Federal University, Brazil ( http://www.unipampa.edu.br/ ).
I’ve just seen the nice collection of infographics
http://www.compoundchem.com/
. It is a very interesting idea.
I’d like to ask you permission to translate to Portuguese, in way
to make available to Brazilian teachers – and other
Portuguese-speaking people
And I need access to the original infographic files (vector graphics).
My website (and weblog) aiming at promoting science and education.
http://www.emsintese.com.br/
and
http://www.tabelaperiodica.org
Thanks in advance.
Prof. Dr. Luis Brudna
luisbrudna@gmail.com
—
ayesha abbas
grt……………
adil nawaz
great
Fran Suarez
I have a question related: which type of isomerism exists between 2-methylpentane and 3-methylpentane, chain or position? Thanks
Тетяна Галенова
Position
Comments are closed.