It’s reaching that point in the year where warm weekends mean it’s time for barbecues out in the sun. Here’s a topical graphic,…


It’s reaching that point in the year where warm weekends mean it’s time for barbecues out in the sun. Here’s a topical graphic,…

As the chemistry of chocolate is a topic that’s been pretty much exhausted on the site (see here, here, here, here, and here), for the Easter weekend we’re instead homing in on the ‘egg’ side of Easter Eggs. For such a simple staple of the kitchen, the chemistry of eggs is surprisingly complex. Here we take a brief look at their composition, and also at some chemistry tips that can help with cooking them!


It’s been a while since the last news on the book, but it’s still very much on its way – and here’s the cover to prove it! It’s out in the UK on October 8, 2015, and it’s already available to preorder on Amazon here. A little more information follows if this is the first you’ve heard about it!


Field-grown rhubarb will shortly be coming into season and appearing in supermarkets in the UK, so it seems like a good time to take a look at the chemistry behind this odd-looking vegetable. It’s mostly used in pies and desserts, but it’s only the stalks of the plant that we eat – and there’s a reason for that. This graphic takes a look at why, and also looks at the chemical compounds that contribute to the colour and the laxative effect of rhubarb.


There’s one chemical reaction that, whether you have an interest in chemistry or not, we all carry out on a regular, maybe even daily, basis. That reaction? The Maillard Reaction. This is a process that takes place whenever you cook a range of foods – it’s responsible for the flavours in cooked meat, fried onions, roasted coffee, and toasted bread. The reaction’s name is a little deceptive, because it’s really an umbrella term for a number of reactions that can produce a complex range of products. The main stages, and some of the different classes of products, are summarised in this graphic.

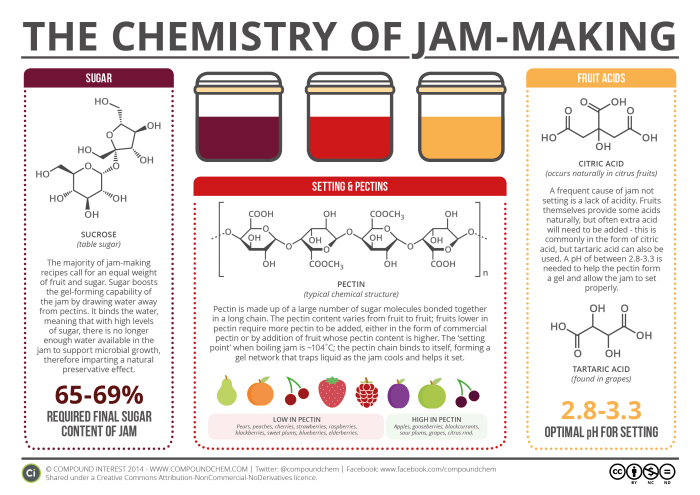
If you’ve ever tried your hand at jam-making, you’ll know that it’s something of a tricky process. A number of factors need to be just right to achieve a perfectly set jam – and chemistry can help explain why. There are three key chemical entities that go into jam-making: sugar, pectin, and acids. Here, we’ll look at each in turn, and how they help jam achieve its eventual consistency.