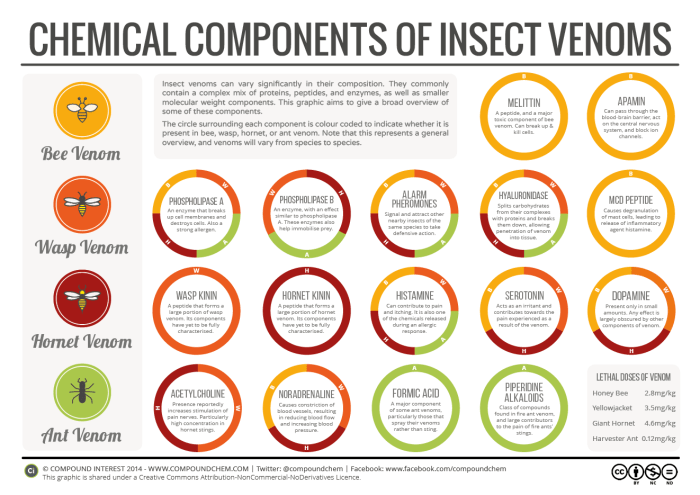
Insect venoms are complicated. Really complicated. You could be forgiven for thinking that it must be a relatively simple company of chemicals that makes up the painful sensation of a bee or wasp sting, but in fact a hugely complex mixture of all sorts of compounds – proteins, peptides, enzymes, and other smaller molecules – go into a small amount of venom. The range of compounds is far too vast to detail every single one – but we can examine some of the major constituents in bee, wasp, hornet and ant venom.
We’ll start with the venom about which we know the most – that of bees. Unlike many other insect venoms, we have a relatively good idea of the percentage breakdown of the venom of your average bee. When the bee stings, the venom is mixed with water, so the actual composition of the substance it injects into you is around 88% water and 12% venom. From this point onward, we’ll consider the percentages of compounds purely in the venom itself.
The main toxic component of bee venom, also referred to as apitoxin, is melittin. Melittin is a peptide that comprises around 50-55% of dry venom, and is a compound that can break up cell membranes, resulting in the destruction of cells. However, it’s not considered the most harmful component of bee venom; that prize goes to an enzyme that makes up around 10-12%, phospholipase A. This enzyme destroys phospholipids, and also breaks down the membranes of blood cells, resulting in cell destruction; additionally, unlike the majority of larger molecules in the venom, it causes the release of pain-inducing agents. Yet another enzyme, hyaluronidase, aids the action of the venom by catalysing the breakdown of protein-polysaccharide complexes in tissue, allowing the venom to penetrate further into the flesh.
Other, smaller molecules can also contribute towards painful effects. A small amount of histamine is found in bee venom; histamine is one of the compounds released by the body during the allergic response, and can cause itchiness and inflammation. The proteins in the sting can cause an allergic reaction, leading to the release of even more histamine, and possible anaphylaxis. MCD peptide, another minor component of the venom, can also cause mast cells in the body to release more histamine, worsening inflammation.
The precise composition of wasp and hornet venom isn’t as well known as that of bees, but we still have a decent idea of what the major components are. The peptides that are found in the venoms are termed ‘wasp kinin’ and ‘hornet kinin’ respectively; these aren’t as well characterised as the peptides in bee venom, however. Like bee venom, they also contain phospholipase A, the enzyme hyaluronidase, and histamine. There are, though, some differences in the chemical composition. As well as variations in percentages of the different components, they also contain the compound acetylcholine, not commonly found in bee venoms. Acetylcholine is actually a neurotransmitter that’s also produced in our bodies, but in wasp and hornet venom, it helps stimulate pain receptors, heightening the pain felt from the sting and venom. Hornet venoms contain particularly high levels of acetylcholine.
You might have been told back in your science classes that bee stings are acidic, and can be neutralised with an alkali, whilst wasp stings are alkaline, and can therefore be neutralised with an acid. Sadly, this is something of an over-simplification. Whilst it’s correct that bee venom has some acidic components, whilst wasp venom has some alkaline constituents, the venom quickly penetrates the tissue once you’ve been stung. Therefore, topical application of an acid or alkali to the sting area is unlikely to provide relief. Additionally, since the venom is such a complex mix of components, many of which have contributing effects, it’s unlikely that neutralising a small number of these components would relieve the pain. What might have some effect, however, is anti-histamine cream, which can help prevent further inflammation.
Whilst there is, of course, variation in venoms between different species of bees, wasps, and hornets, in ants this is markedly the case. The venom of some ants contains very little protein and peptide content, and is composed instead mainly of smaller compounds. An example is that of the fire ant. Fire ant venom consists of only around 0.1% of the dry venom, with the vast majority instead consisting of a class of compounds called alkaloids; these alkaloids are toxic to cells, and result in a burning sensation. Although the protein content is much lower than that of bees, wasps, and hornets, it can also cause allergic reactions and anaphylaxis.
Other ants don’t sting, but can instead spray their venoms; amongst many the main constituent of venom is formic acid. This leads us to a chemical reaction that is worth talking about. As it turns out, as unpleasant as the venom of the fire ants is, they meet their match in another species of ant, the ‘tawny crazy ant’. These two warring species of ants both make use of their venoms in conflict, but the tawny crazy ant uses chemistry to gain a clear advantage. They combat the toxicity of fire ant venom by detoxifying it with their own, which is based on formic acid. Researchers still don’t fully understand precisely how the detoxification occurs, but suggest it might be the result of the formic acid neutralising the enzymes that aid in fire ant venom’s potency. Even more interestingly, this detoxification process forms an ionic liquid at ambient temperature, a phenomenon that had not previously been observed in nature.
A final word on venoms goes to a component that is present in all four of the venoms we’ve considered: alarm pheromones. As if being stung by a bee or hornet wasn’t bad enough, the pheromones contained in the venom (which tend to be a complex mix of volatile low molecular weight compounds) signal to other members of the same species to take defensive action. In plain English, a wasp stinging you signals to other wasps that they should grab a piece of the action too. Apparently, the odour of the bee pheromone is reminiscent of bananas, though it’s probably not a theory you want to investigate.
EDIT: Bonus graphic! This one looks at the Schmidt Pain Index, developed by entomologist Dr. Justin Schmidt to rank the pain of the various insect stings and bites he experienced as part of his work. Whilst both the pain of a sting and its duration is subjective, and these rankings therefore may not hold true for everyone, it’s still an interesting ranking to look at. If there’s one thing that’s apparent from this graphic, it’s ‘never get stung by a bullet ant’!

The graphic in this article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. See the site’s content usage guidelines.
References & Further Reading
- Bee & Wasp Venoms – E Habermann
- Hymenopteran Envenomation – J Schmidt
- Bee Venom – S Bogdanovic
- The Chemistry of Bees – University of Bristol
- Ant Venoms – D R Hoffman
- Visual Guide to Painful Insect Stings – Joshua Stevens






21 Comments
O.R. Pagan
Nice article! Just one detail though. If I remember correctly hyaluronidase degrades certain carbohydrates, not proteins…
Compound Interest
Thanks! It seems the reference I pulled that information from might have been over-simplifying to the point of being a little wrong. From what I can gather, the polysaccharides that hyaluronidase breaks down are often complexed with proteins, so it also assists the breakdown of these protein-polysaccharide complexes – which is where I’m guessing the reference’s description of it ‘hydrolysing proteins’ may have come from?
Either way, it seems it would be more accurate to state that it breaks down protein-polysaccharide complexes and certain carbohydrates; that, or simplifying it further to state that it breaks down components of the intracellular matrix?
O.R. Pagan
Sorry for taking so long to answer; I have to check discuss more… You are absolutely right!
disqus_q66LTHDXxQ
It would be most helpful to know where this bullet ant lives, so I can avoid it…where do the other stingers live?
John Milligan
According to Wikipedia, it lives on the rain forests from Nicaragua & Honduras down to Paraguay.
Another part of the world to avoid?
Garrick Burron
In your second figure you list “Velvet Ant” but the graphic implies it’s a wasp. Is this an error?
Compound Interest
Oddly enough, it isn’t – the “Velvet Ant” is, despite the name, a type of wasp. I actually also assumed it was an ant (you would, wouldn’t you?) and had it indicated as such on an initial version of the graphic, before hastily correcting it!
mimi boothby
how do you destroy apitoxin? I can’t find any information on this, in fact, beekeepers wash their beekeeping clothing separately as to not get apitoxin on their family’s clothing thanks
Comments are closed.