If you’ve ever tried your hand at jam-making, you’ll know that it’s something of a tricky process. A number of factors need to be just right to achieve a perfectly set jam – and chemistry can help explain why. There are three key chemical entities that go into jam-making: sugar, pectin, and acids. Here, we’ll look at each in turn, and how they help jam achieve its eventual consistency.
Pectins
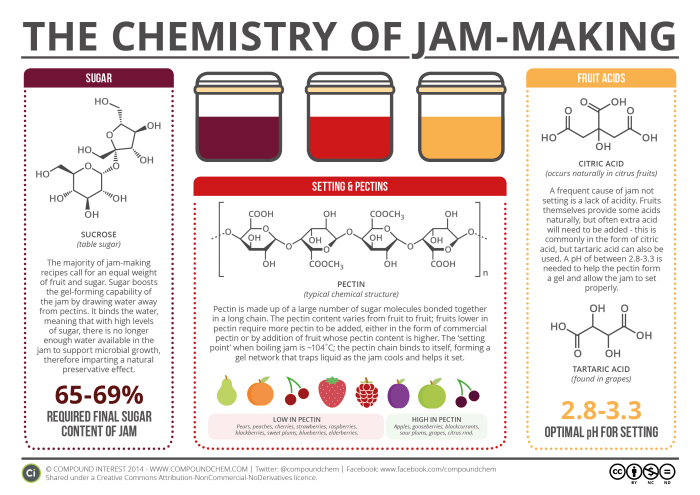
Pectins are long, linked chains of sugar molecules, which are found naturally in plant cell walls. Although we refer to them in general as ‘pectin’, their structures are variable, as well as hard to determine; a rough general structure is given in the graphic, but in reality the overall structure can be much more complicated. Pectins are found in fruits, particularly in the peels and cores. When jam sets, pectin plays a vital role.
Boiling the jam releases the pectins from the fruit used; with the correct amount of sugar and acidity, which we’ll discuss in due course, the long pectin chains can bind to each other via intermolecular interactions, forming a gel network. This network generally forms at the ‘setting point’ of jam, which is approximately 104˚C. Once it has formed, the jam can be allowed to cool, and the gel network ‘traps’ the water content of the jam, leading to setting.
The pectin content of different fruits varies: fruits such as apples and blackcurrants have higher levels of pectin than those such as strawberries and raspberries. In cases where a jam is being made from a low pectin fruit, either a higher pectin fruit must also be included, or commercial pectin must be added. Commercial pectin is obtained from the peel of citrus fruits, which have a naturally high pectin content.
Sugar
An important part of jam is, of course, the sugar content, which is vital for the flavour and also plays a role in helping jam set. Many jam recipes recommend the use of a 1:1 ratio of fruit to sugar in jam-making. As well as sweetening the jam, the sugar also helps the pectin set – it enhances the pectin’s gel-forming capability by drawing water to itself, decreasing the ability of the pectin to remain in separate chains. Additionally, sugar imparts a preservative effect. By binding water molecules to itself, it reduces the amount of water available in the jam, to the point at which it is too low for microbial growth, helping to ensure that the jam doesn’t go off too rapidly after it’s been made! The final sugar content of jam should be between 65-69%.
Acids
Acids are also important in helping the pectin to set. The COOH groups in the pectin are usually ionised, and the negative charges on the molecules this ionisation causes can cause repulsion, and prevent the pectin chains from being able to form the gel network. To avoid this, we need the pH of the mixture to be roughly in the range of 2.8-3.3. At these more acidic pHs, the COOH groups aren’t ionised, lowering the magnitude of the repulsive forces.
Fruits naturally contain acids – the most well known is citric acid, but malic acid and tartaric acid are also found in a number of fruits. Whilst some acid will be contributed by the fruit from which the jam is made, often this won’t be enough to reach the desired pH, and for this reason more must be added. This is commonly in the form of lemon juice, which contains citric acid, though powdered forms of acids can also be used.
In summation, then, the three factors of pectin, sugar and acid have to be in perfect balance for jam to set. If it doesn’t, you can often point to one of those three factors being somehow amiss – and understanding the chemistry behind why jam sets in the first place can often help you identify how to fix it!
A modified version of this graphic will be included in the upcoming book “Why Does Asparagus Make Your Wee Smell?”, available to pre-order now!
The graphic in this article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. See the site’s content usage guidelines.
References & Further Reading
- The Science & Magic of Jam-Making – Guardian Science
- Jam Science – Scientific Explorer
- Chemistry of Pectin – P Sriamornsak
- Science of Jams & Jellies – Dr M Bourne






14 Comments
Ana
Great article!
Compound Interest
Thanks Ana, glad you enjoyed it!
satish
what about the pectins that allow for low or no sugar? strange they have added SODIUM CITRATE and FUMARIC ACID, how does this pectin get thick when there is no sugar? And why cant they add citric acid?
satish
is is there any heat needed. i seen the videos of the them cooking the jam?
clothespin
Thank you so much for this! As a botanist (well, now a mommy) and a home canner, this has come in handy. I’m working on a recipe for hibiscus tea jelly… and thanks to a ph test in the lab and your information, I at least have a starting point on where to go to make the recipe work. (The tea is super acidic so am going to try adding another juice to raise it up.) As I tell my girls… cooking is just science that you can eat!
Compound Interest
You’re welcome! If you’re into canning, this graphic might also be of interest: http://cen.acs.org/articles/94/i36/Periodic-graphics-chemistry-canning.html
Comments are closed.